-
Leriglitazone
- What is Leriglitazone
Leriglitazone (MIN-102) is a novel, orally bioavailable and selective PPAR gamma agonist with a potential best-in-class profile indicated for CNS diseases. It is one of the several metabolites of pioglitazone and has a demonstrated sufficient brain penetration and favorable safety profile in humans, allowing PPAR gamma engagement in the CNS above the level that can be safely achieved with pioglitazone and other glitazones.
In X-ALD clinical trials, leriglitazone has demonstrated efficacy in adult ALD patients (ADVANCE) and in paediatric patients with cerebral ALD (NEXUS) overall showing clinical benefit in these populations. A US registration study in adult male progressive cALD patients (CALYX), is currently recruiting.
Leriglitazone showed robust preclinical proof-of-concept in animal models of multiple other diseases, including Rett and NBIA, by modulating pathways leading to mitochondrial dysfunction, oxidative stress, neuroinflammation, demyelination and axonal degeneration.
1 Best-in-class, selective PPAR gamma agonist Central role in CNS. Metabolite of pioglitazone2 Leriglitazone has demonstrated efficacy in phase 3 and the MAA is currently under review with EMA3 In the clinic leriglitazone is generally well tolerated without significant safety findings4 Orally bioavailable. Specifically formulated as suspension to achieve high and controlled exposure in patients5 MoA validated in animal models of multiple CNS diseases6 Granted patents in European jurisdictions and US covering multiple CNS indications until 2039
Mechanism of Action in X-ALD
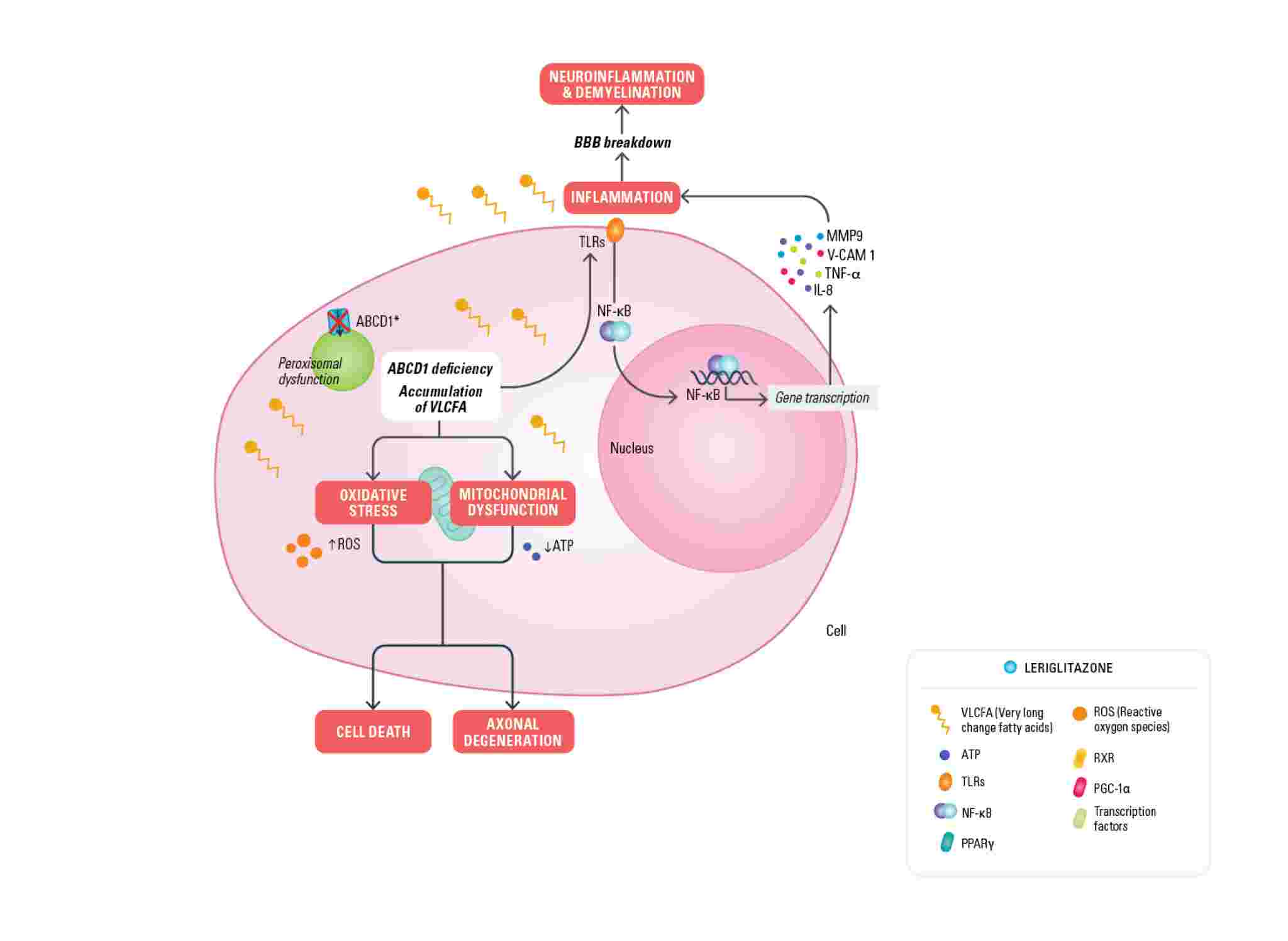
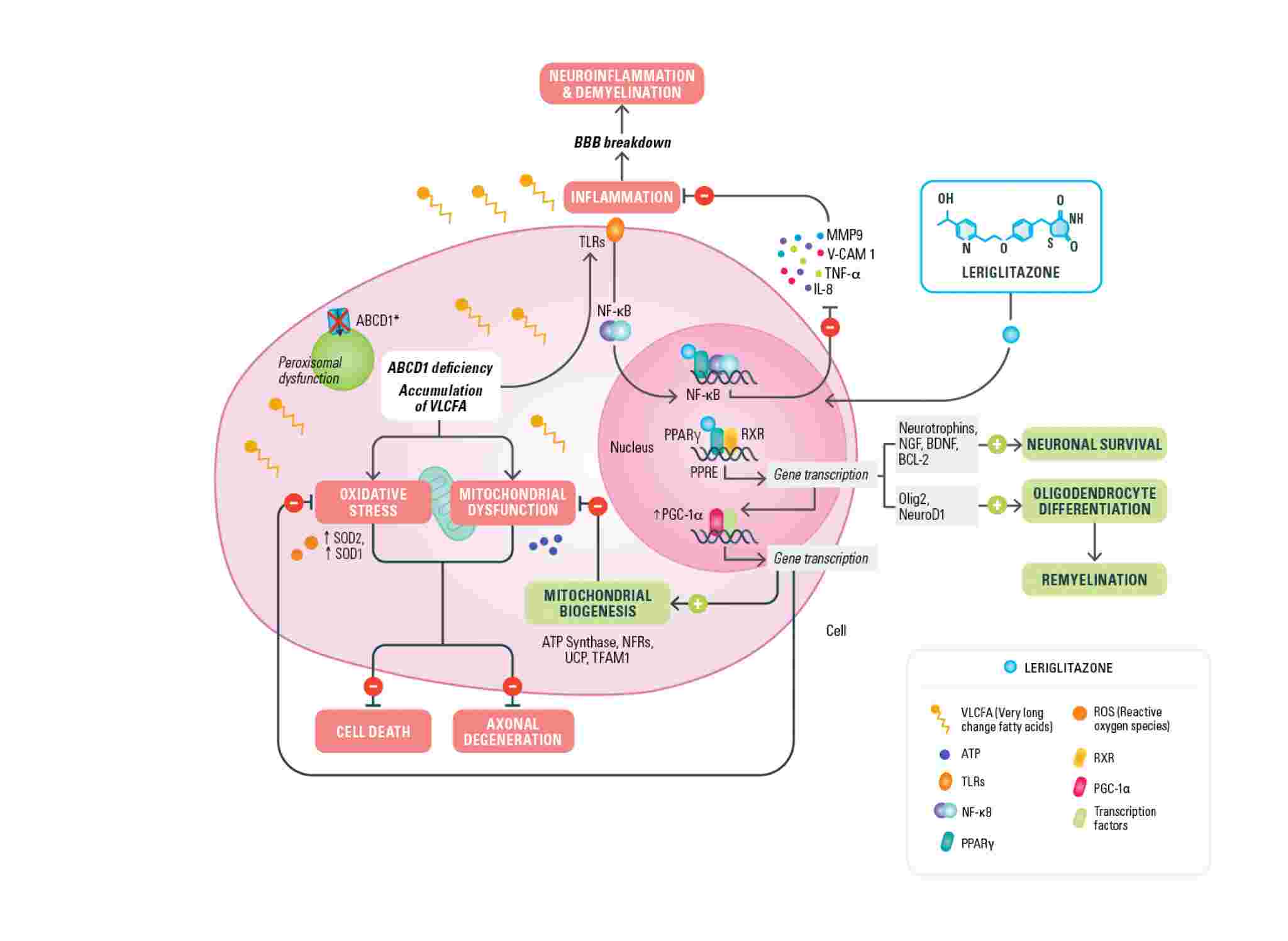
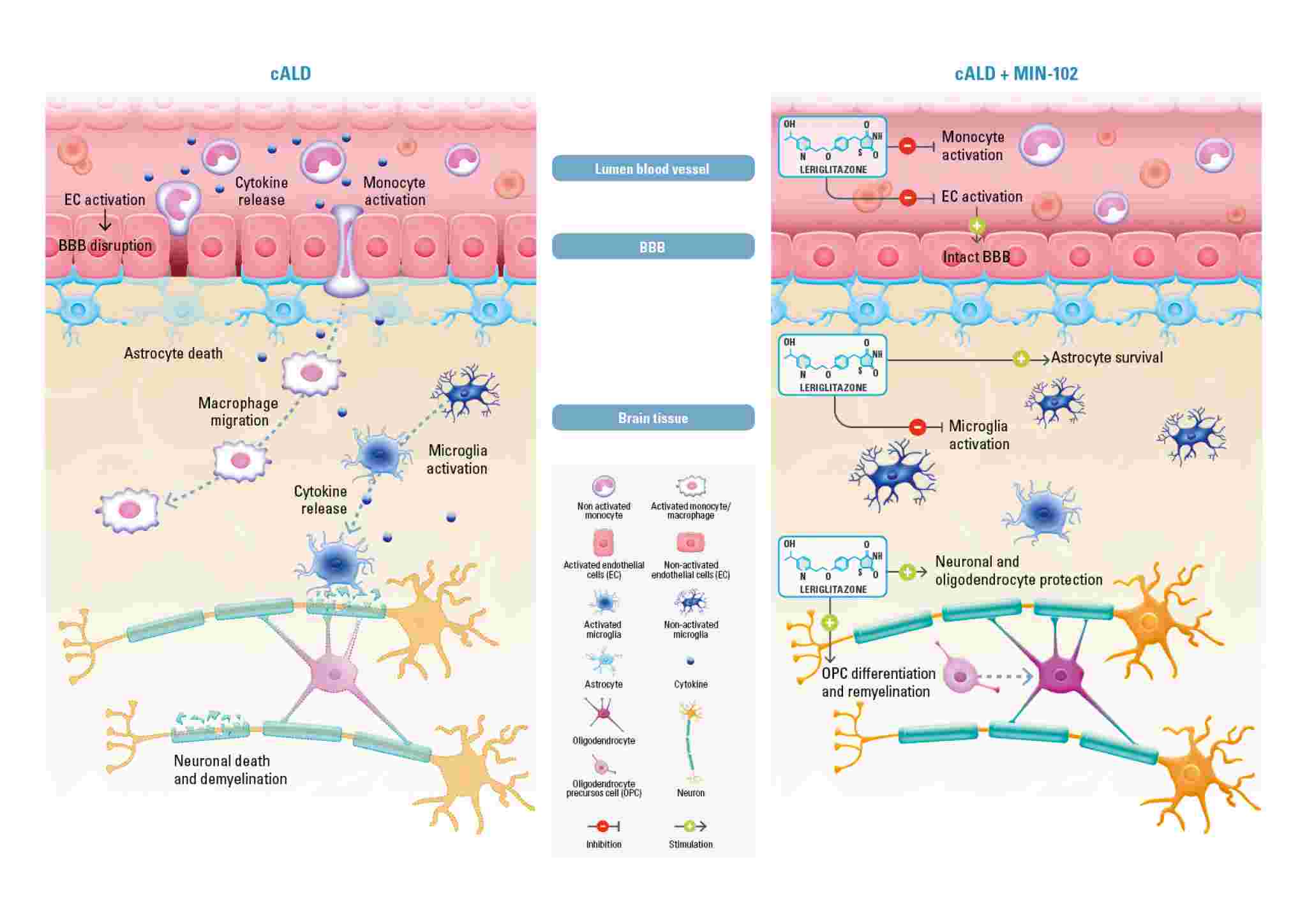
In X-ALD, mutations in the ABCD1 gene located on the X-chromosome cause defective function of the ABCD1 transporter, normally responsible for bringing VLCFAs (very long-chain fatty acids) into the peroxisome. The defective function of the transporter leads to an accumulation of very long-chain fatty acids (VLCFA) in several tissues and a pathogenic cascade of events that contribute to membrane destabilization of the myelin sheath, mitochondrial dysfunction, oxidative stress, neuroinflammation and compromised blood brain barrier (BBB) integrity.
Leriglitazone (MIN-102), by activating PPAR gamma, modulates the expression of genes involved in mitochondrial biogenesis (PGC-1a) and therefore restores the lost energy balance, decreases oxidative stress and restores mitochondrial function caused by the accumulation of VLCFAs. Leriglitazone also increases the expression of genes involved in oligodendrocytes differentiation (olig2, neuroD1) promoting remyelination by oligodendrocyte survival and differentiation and modulates neurotrophin levels resulting in improved neuronal survival. Leriglitazone also interacts with the inflammatory pathway active in X-ALD by decreasing NF-kB levels, reducing macrophage/microglial activation and consequently neuro-inflammation. It also reduces monocyte adhesion to the endothelial cells of the blood brain barrier, a mechanism that leads to BBB disruption and has a prominent role in the initiation of the cALD phenotype. The mechanism of action of leriglitazone in X-ALD was published in Science Translational Medicine Journal.
Mechanism of Action in other CNS diseases
FRDA
Friedreich's Ataxia caused by gene mutations which result in deficient expression of the mitochondrial protein frataxin (FXN) and subsequent changes in mitochondrial function. In particular mitochondrial iron metabolism is affected causing iron accumulation, mitochondrial dysfunction and oxidative damage. Frataxin is highly expressed in cardiomyocytes and in dorsal spinal column neurons. The maintenance of mitochondrial homeostasis is crucial during the progression of the disease.
Through the activation of the PPAR gamma/PGC-1 alpha pathway, leriglitazone restores mitochondrial function and energy production. This results in increased neuronal survival and decreased neurite degeneration and improvement of motor function in animal models of the diseases. Preclinical data for leriglitazone in FRDA was published in the Neurobiology of Disease Journal.